What is Leprosy?
Hansen’s Disease
Leprosy, also known as Hansen’s Disease is a chronic,mildly communicable disease caused by infectiona from Mycobacterium leprae, a rod-shaped, acid-fast bacillus. It primarily affects the skin; the mucous membranes, especially those in the nose; and the peripheral nervous system.
Leprosy exacts a high physical and social toll. If left untreated, it can result in deformity and disability. In many societies, people with leprosy have been made outcasts from their communities

The Cause
The Norwegian doctor, Gerhard Armauer Hansen first observed M. leprae as an acid-fast, alcohol-fast, strong Gram-positive bacillus in tissue specimens from leprosy patients in 1873. The following year, he proposed that the bacteria caused leprosy; hence the name ‘Hansen’s Disease’ emerged.

Transmission
M. leprae infection occurs primarily in human beings. Researchers do not know exactly how the bacteria are transmitted. For many years, leprosy was believed to be transmitted through skin-to-skin contact. However, experts now consider this unlikely because M. leprae are not usually found on the skin’s surface. Most evidence suggests that people become infected by inhaling the bacteria. With each cough or sneeze of an untreated Person With Leprosy (PWL), the bacilli are discharged as droplets. Prolonged, close contact with infectious persons is likely to increase the risk of transmission.
Susceptibility
While the leprosy bacilli may be transmitted easily, perhaps 95 percent of people who are infected do not develop the disease. Cell-mediated immunity (CMI), or the body’s ability to resist infections, is the mechanism that protects against leprosy. Most people who are exposed to M. leprae resist infection and develop immunity after this exposure.
Only a few actually develop the disease. Symptoms of leprosy typically appear three to five years after infection.
Diagnosis
Diagnosis of leprosy is commonly based on clinical signs and symptoms. Only in rare instances is there a need to use laboratory and other investigations to confirm a diagnosis of leprosy. A person should be regarded as having leprosy if he exhibits one or more of the following cardinal signs:
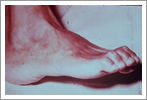
1. Whitish (hypopigmented) or reddish patches of skin called skin lesions with loss of feeling
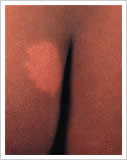
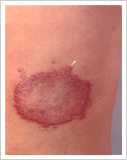
2. Damage to the peripheral nerves as demonstrated by loss of sensation and weakness of the muscles of the hands, feet and/or face;

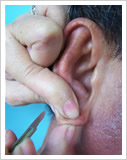
3. Skin smears that are positive for M. leprae

Classification
The World Health Organization (WHO) has three (3) classifications of leprosy:
Single Lesion Paucibacillary (SLPB). Identified by only one (1) leprosy lesion, whose surface may either be normal, dry or scaly, with some degree of loss of sensation. Border may be ill-defined or well-defined, and slit skin smears are negative for M. leprae.
Paucibacillary (PB). Two to five infiltrated patches, whose surface may be normal, dry or scaly with absence of hair growth. There may either be partial or total loss of sensation. Borders are well-defined and skin smears are negative for M. leprae. Peripheral nerves may be affected
MultiBacillary (MB). More than five macules, plaques, papules or infiltrated patches, whose surface may be dry, smooth and shiny. Outer borders are vague, sloping outwards and merging imperceptibly with surrounding skin. Sensation may be normal or slightly diminished. More than one peripheral nerve is affected, and skin smears are always positive for M. leprae.
Treatment
The drug of choice of the National Leprosy Control Program (NLCP) is the WHO-recommended Multi-Drug Therapy (MDT). It is a combination of two or more of the following drugs:Clofazimine, Dapsone, Ofloxacin and Minocycline.
MDT is fast-acting and prevents the emergence of drug-resistant strains of M. leprae.
Paucibacillary (PB) Regimen
Dapsone given in 28-day monthly blister packs. Treatment is completed when the patient has taken six packs within a maximum of 9 months.


Multibacillary (MB) Regimen
Clofazimine and Dapsone given in 28-day monthly blister packs. Treatment is completed when the patient has taken twelve packs within a maximum of 18 months.